A high barrier to drug resistance should be a major consideration
Replication of the HIV-1 virus is error-prone, resulting in millions of mutations each day. When mutations occur in virus proteins that are the targets of antiretroviral (ARV) drugs, drug resistance can occur.1-3
Types of drug resistance
There are two types of drug resistance: transmitted and treatment-emergent (also called therapy-emergent) resistance.4
Transmitted resistance
Transmitted drug resistance occurs when a drug-resistant virus is transmitted from one person to another.4 According to the DHHS guidelines:
- Transmitted drug-resistant HIV is generally either NNRTI- or NRTI-resistant5
- Transmitted PI resistance is much less common than either NNRTI or NRTI resistance and, to date, INSTI resistance is rare5
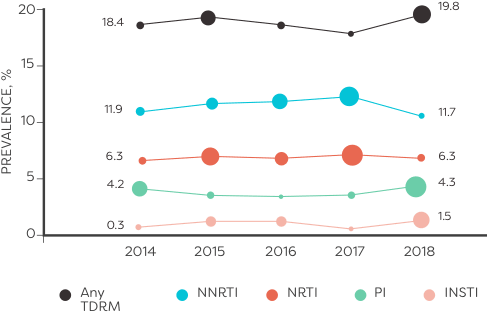
In an analysis of HIV sequences reported to the National HIV Surveillance System from 2014 to 2018 (n=50,747):
ALMOST 10,000 TREATMENT-NAÏVE PATIENTS had transmitted resistance mutations to ≥1 class of ARVs6
Treatment-emergent drug resistance
Treatment-emergent drug resistance occurs when the HIV-1 virus replicates in the presence of suboptimal or inconsistent levels of ARVs. Adherence to a prescribed ARV regimen is necessary to maintain virologic suppression.4,5,7
Consider the impact of suboptimal drug levels4,7,8
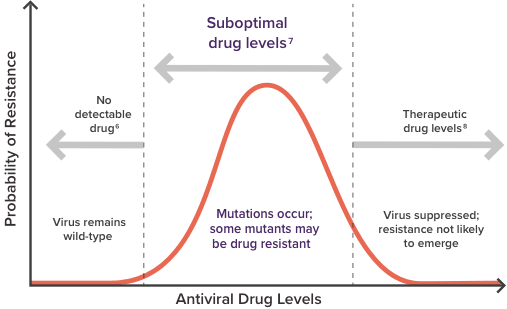
The likelihood of developing drug resistance is dependent on the level of ARVs in the plasma while the HIV virus is replicating.4,7,8
The main factors that contribute to suboptimal ARV plasma levels include:
Poor treatment
adherence9-11
Food effects9
Drug interactions10,11
Understanding an ARV’s barrier to resistance
An ARV's barrier to resistance refers to the threshold above which clinically meaningful resistance develops (or the ease with which resistance develops) to a given drug (and in some cases, to other drugs in the same class). Resistance barriers for specific ARVs vary from low to high, based on the interplay among key contributing factors12,13:
-
ARV genetic barriers12
-
The number of critical mutations needed to make an ARV ineffective
-
-
Susceptibility and/or viral fitness of drug-resistant HIV7,12
-
Preexisting resistance can impact viral replication and the efficacy of the drug being used to varying levels
-
-
There is essentially no cross-resistance among ARV drug classes (ie, resistance to one class does not impact the activity of other classes of unused ARVs)14

Reducing the risk of resistance
- Durable virologic suppression, which can help prevent the development of resistance, has driven the shift away from monotherapy recommendations toward regimens with a variety of drug classes to suppress HIV1,15
- A more complex regimen may be required if new resistance mutations emerge. These new mutations can be caused by virologic failure.5
Resistance mutations may jeopardize future treatment options. Therefore, taking into account the lifelong complications that your patients with HIV may face as a result of resistance should be a major consideration during treatment selection.5
For illustrative purposes only. Not actual physician.
HIV drug resistance testing
Genotypic, phenotypic, and proviral DNA tests are used to detect drug-resistant HIV viral strains.5
Genotypic resistance assay predicts drug susceptibility by comparing a patient’s viral DNA sequence and a wild-type reference sequence.5
Phenotypic resistance assay exposes a sample of the patient’s HIV to all available ARVs at different concentrations and measures the virus’s ability to replicate. The test can determine which drugs will work and which no longer respond in the current ARV regimen.5
An HIV-1 proviral DNA test in host cells is now commercially available. This test is intended to detect archived resistance mutations in patients with HIV RNA below the limit of detection or with low-level viremia. It can be an option when standard resistance testing is unsuccessful due to inadequate HIV-RNA levels.5
Consider prior and current drug resistance test results when evaluating comprehensive therapies for your patients.5
DHHS guidelines
Resistance testing in treatment-naïve adults5
- ART initiation should not be delayed while awaiting resistance test results; the HIV regimen can be modified once results are reported. Early treatment initiation should be administered in persons with acute or recent (early) HIV infection, in pregnant people with HIV, or in people who will initiate ART on the day of or soon after diagnosis5
- Baseline resistance testing: recommended for people with HIV at entry into care to guide selection of the initial ART5
- Standard genotypic testing: involves testing for mutations of the reverse transcriptase (RT) and protease (PR) genes. If transmitted INSTI resistance is a concern, providers should ensure that testing also includes the integrase gene5
Resistance testing in ART-experienced adults
In cases of virologic failure:
-
HIV drug-resistance testing should be performed to aid in selection of active drugs when switching ART regimens in:
- People with virologic failure and HIV RNA levels >1000 copies/mL5
- People with suboptimal viral load reduction5
- In cases of virologic failure, perform HIV drug-resistance testing while the person is taking prescribed ARVs, or if that is not possible, within 4 weeks after discontinuing therapy5
- When a person experiences virologic failure while receiving an INSTI-based regimen, genotypic testing for INSTI resistance should be requested5
In cases of virologic suppression:
(ie, switches due to adverse events, drug-drug or drug-food interactions, pill burden, pregnancy, cost, or the desire for regimen simplification)5
- It is critical to review a patient’s full ARV history, including virologic responses, past ARV-associated toxicities and intolerances, and cumulative resistance test results before selecting a new ART regimen5
- Within-class and between-class switches can usually maintain viral suppression, provided there is no viral resistance to the ARV agents in the new regimen5
- Monotherapy with either a boosted PI or an INSTI has been associated with unacceptable rates of virologic failure and the development of resistance; therefore, monotherapy as a switching strategy is not recommended5
Long-term treatment success can potentially be threatened by HIV drug resistance. DHHS guidelines recommend that you consider the importance of a high barrier to resistance during ART selection.5
ART, antiretroviral therapy; DHHS, US Department of Health and Human Services; INSTI, integrase strand transfer inhibitor; NNRTI, non- nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RNA, ribonucleic acid; TDRM, transmitted drug resistance-associated mutations.
References:
- Soriano V, Perelson AS, Zoulim F. Why are there different dynamics in the selection of drug resistance in HIV and hepatitis B and C viruses? J Antimicrob Chemother. 2008;62(1):1-4.
- Cromer D, Schlub TE, Smyth RP, et al. HIV-1 mutation and recombination rates are different in macrophages and T-cells. Viruses. 2016;8(4):118-132.
- AIDSMap. Resistance mutations. Updated July 2019. Accessed February 1, 2022. https://www.aidsmap.com/about-hiv/resistance-anti-hiv-drugs
- Tang MW, Shafer RW. HIV-1 antiretroviral resistance: scientific principles and clinical applications. Drugs. 2012;72(9):e1-e25.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Updated May 26, 2023. Accessed July 24, 2023. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/guidelines-adult-adolescent-arv.pdf
- McClung RP, Oster AM, Ocfemia MCB, et al. Transmitted drug resistance among human immunodeficiency virus (HIV)-1 diagnoses in the United States, 2014–2018. Clin Infect Dis. 2022;74(6):1055-1062.
- Gardner EM, Burman WJ, Steiner JF, Anderson PL, Bangsberg DR. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS. 2009;23(9):1035-1046.
- HIVinfo.NIH.gov. HIV treatment: drug resistance. Updated August 4, 2021. Accessed February 1, 2022. https://hivinfo.nih.gov/understanding-hiv/fact-sheets/drug-resistance
- Atkinson MJ, Petrozzino JJ. An evidence-based review of treatment-related determinants of patients' nonadherence to HIV medications. AIDS Patient Care STDs. 2009;23(11):903-914.
- Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2(4):a007161.
- Gonzalez-Serna A, Swenson LC, Watson B, et al. A single untimed plasma drug concentration measurement during low-level HIV viremia predicts virologic failure. Clin Microbiol Infect. 2016;22(12):1004.e9-1004.e16.
- Luber AD. Genetic barriers to resistance and impact on clinical response. MedGenMed. 2005;7(3):69.
- Zdanowicz MM. The pharmacology of HIV drug resistance. Am J Pharm Educ. 2006;70(5):100.
- Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292-307.
- Pau AK, George JM. Antiretroviral therapy: current drugs. Infect Dis Clin North Am. 2014;28(3):371-402.
